ABOUT US
BIOGENOVA is a privately owned biotechnology company that discovers, develops and manufactures tools and reagents as well provide related services to support scientists in molecular and structural biology, protein biochemistry, genomics, proteomics and drug discovery.
Our experienced research and development team brings the most innovative technologies to your bench via top quality products and solutions.
Our customers include academic and government institutions as well as biotechnology and pharmaceutical companies.
Our focus is on our customer and serving their research needs by enabling them to conduct research more effectively for the rapid expansion of scientific knowledge and discovery of new medicines.

Explore Diverse Refolding Strategies for Solubilized Denatured Proteins
NEW MACROMOLECULE CRYSTALLIZATION SCREENS!
Proteins that are expressed in bacteria frequently form insoluble aggregates called inclusion bodies. These inclusion bodies can be solubilized using buffers with chatropic salts, detergents or low pH. In some cases through dialysis of this material in appropriate in vitro buffer conditions may facilitate the refolding of protein into an active and soluble state.
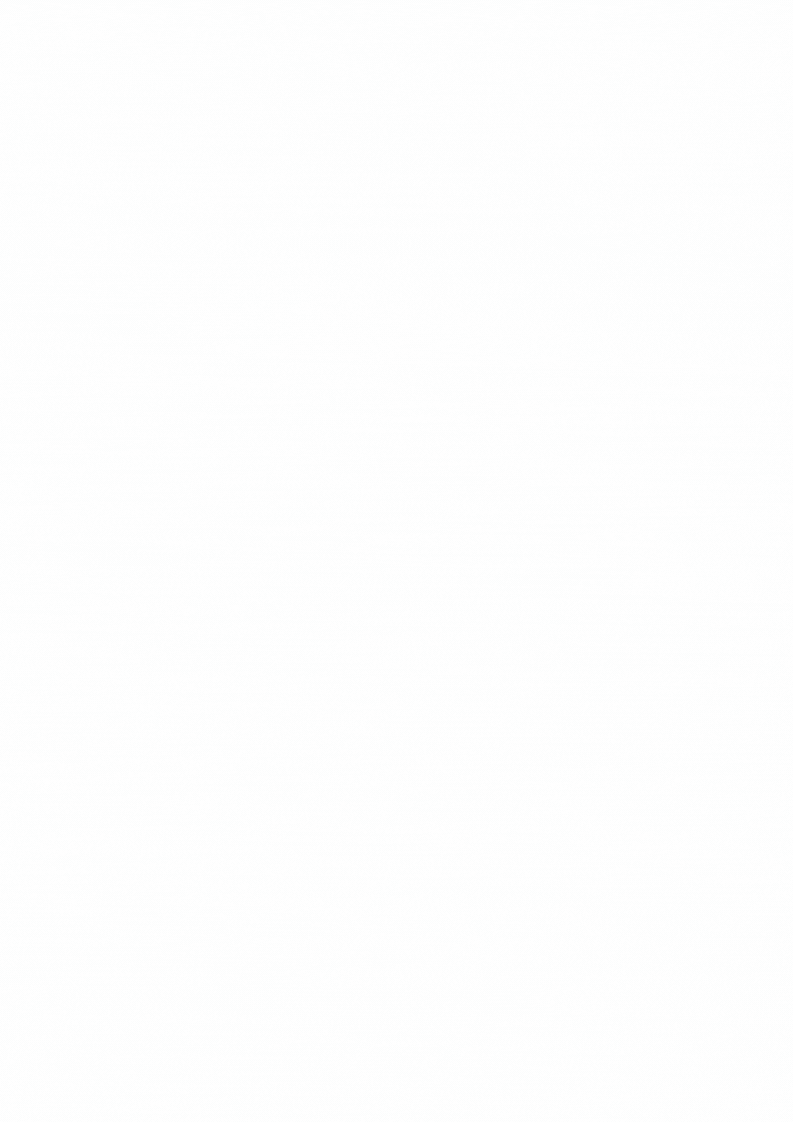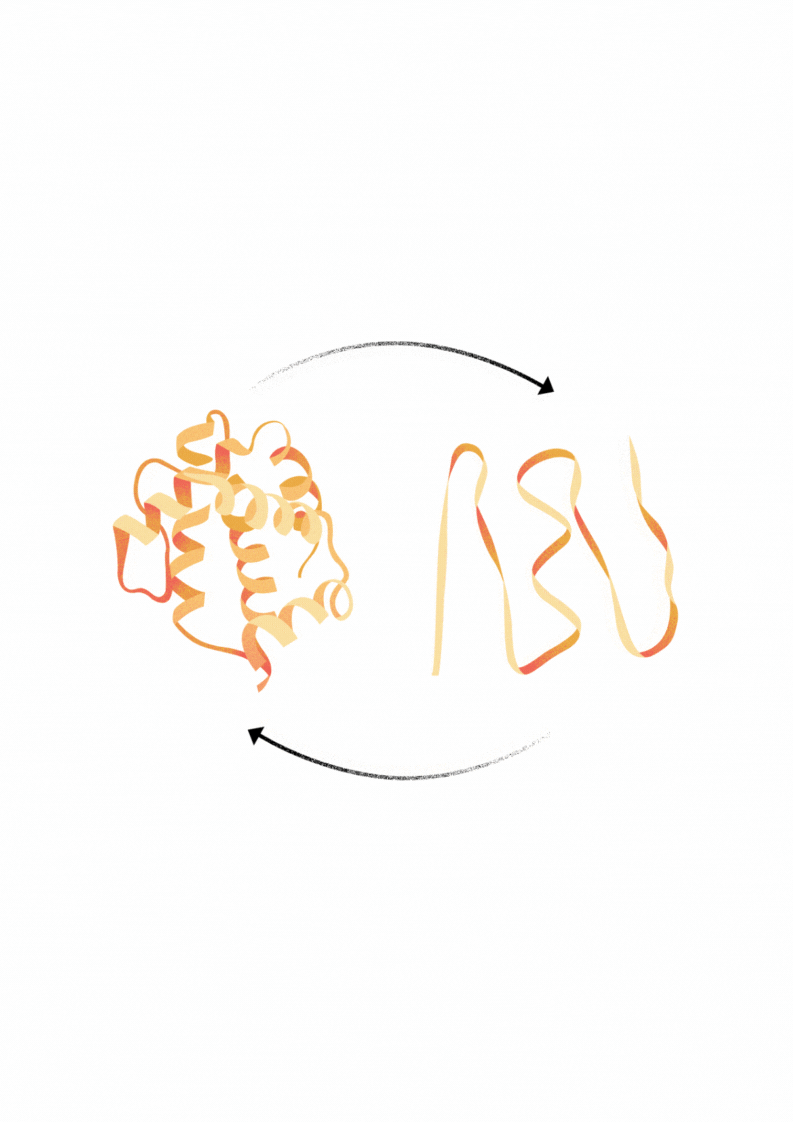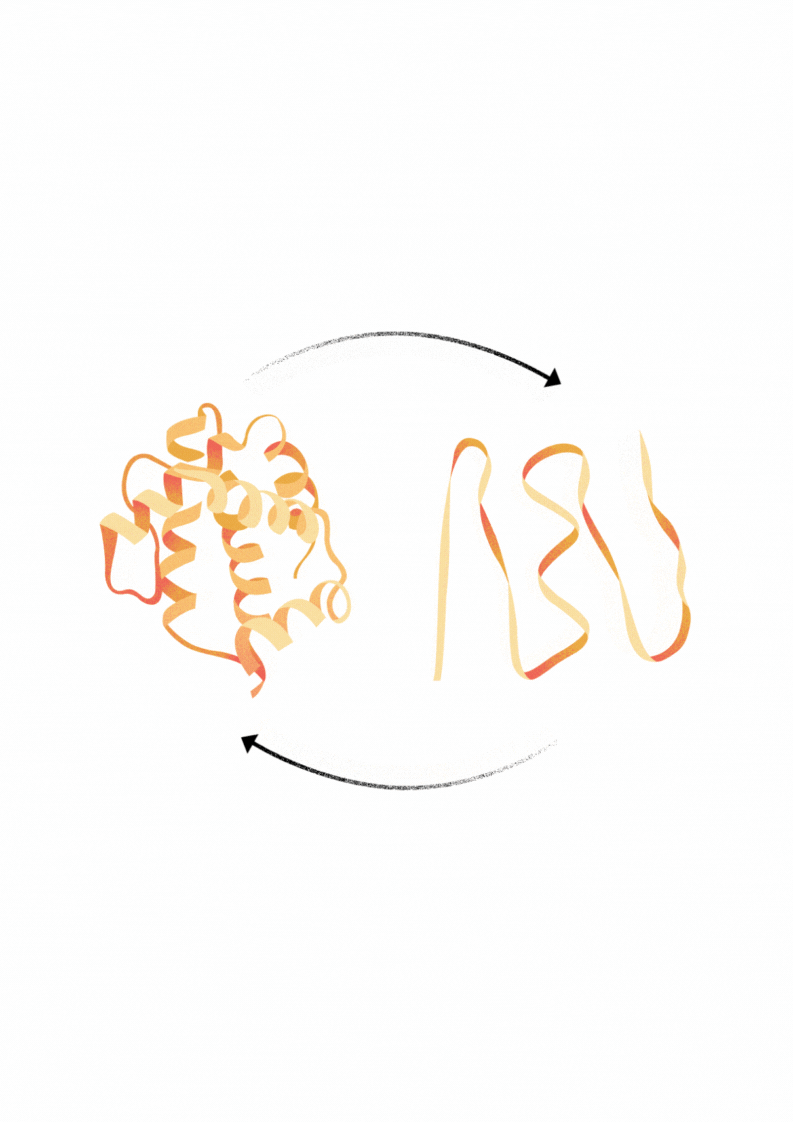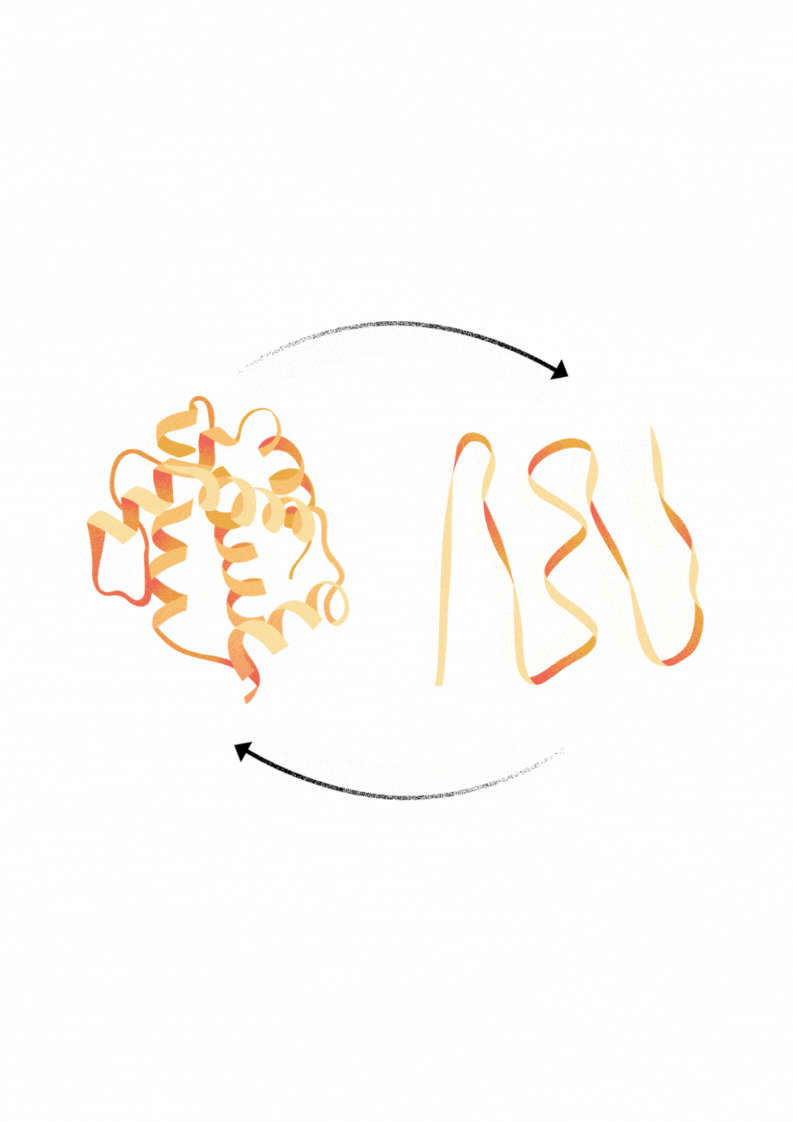
TISSUE MICROARRAYS
One stop for all your Tissue Microarray needs!
Tissue MicroArray (TMA), also called "tissue-chips" is a newly developed technology that provides a way to characterize genes and proteins at the cellular and tissue levels in a high throughput manner. TMA has been used extensively to understand the biological function of genes and proteins, and to identify and validate molecules of diagnostic and therapeutic importance. Backed by strong teams of experienced and dedicated pathologists and scientists, we are a leading provider of tissue microarray products and related services, we have established a comprehensive and integrated system to collect, track, and manufacture hundreds of thousands of tissue microarrays from various normal and diseased human and animal tissues.
The tumor tissues that are currently offered include tumors of lung, breast, kidney, liver, brain, stomach, head and neck, testis, prostate, rectum, ovary, bladder and osteosarcoma and lymphoma.
Learn More
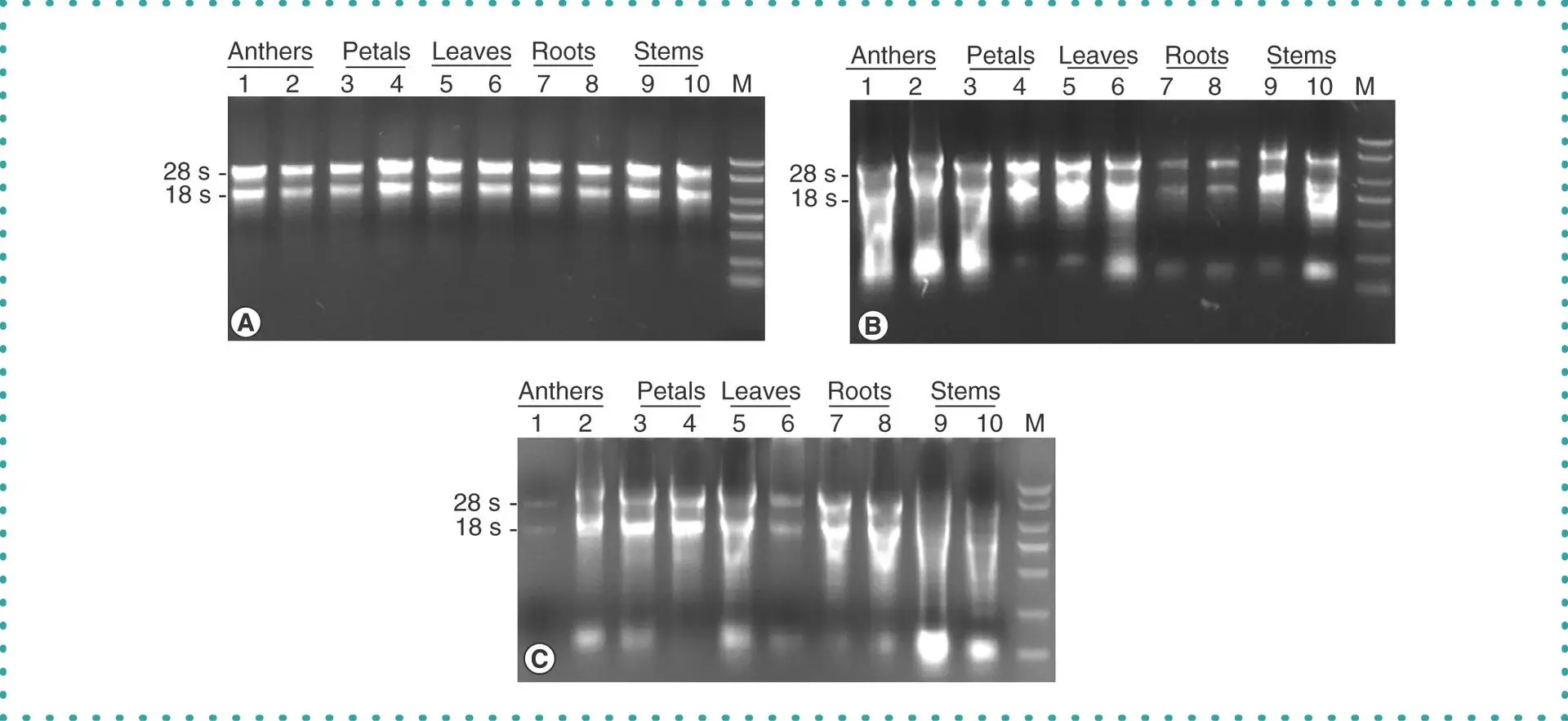
cDNA and Northern Blot Analysis
High-quality cDNA blots prepared from various human, mouse, and rat tissues are also available for gene expression studies. Each membrane is spotted with normalized quantities of cDNA derived from multiple tissue sources, allowing for parallel comparison of gene expression levels across different organs. The blots are immobilized on positively charged nylon membranes and are compatible with both radioactive and non-radioactive detection methods. Internal controls and molecular weight markers are included to ensure accurate hybridization and transcript size estimation.
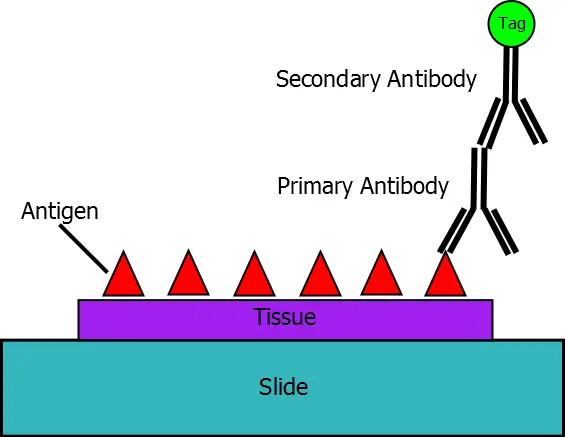
IMMUNOHISTOCHEMISTRY (IHC) & IN SITU HYBRIDIZATION (ISH) SERVICES
The standard immunohistochemistry (IHC) and standard in situ hybridization (ISH) services are performed to localize the protein/gene of interest by using customer-supplied antibodies DNA fragments or oligonucleotides on selected normal and diseased human tissues from our tissue array bank. These services are highly cost effective and are conducted to the highest standards to assist you in understanding the function of your genes/proteins or in identifying and validating potential therapeutic targets. The results are presented as a publishable, detailed pathology report accompanied by high-resolution digital photographs for publication.
Facilities for the Production and Quality Control of Clinical-Grade Vectors
The manufacturing of cell- and gene-based therapies in compliance with Good Manufacturing Practices (GMP) presents a significant challenge for many clinical research teams. Meeting stringent regulatory standards, ensuring product consistency, and maintaining rigorous quality control all require advanced technical capabilities and specialized infrastructure.
To address this growing demand, several high-level production platforms now offer services dedicated to the manufacturing of clinical- and preclinical-grade therapeutic products, including plasmids, synthetic oligonucleotides, and viral vectors. These facilities are also equipped to perform a wide range of safety and quality control tests necessary to meet regulatory requirements.
Through close collaboration with these certified manufacturing centers, we actively support the translation of advanced biotherapies into clinical settings facilitating access to high-quality therapeutic solutions while ensuring compliance with all applicable standards. Our approach enables a smooth transition from development to delivery, reinforcing the global effort to accelerate innovation in drug discovery and precision medicine.
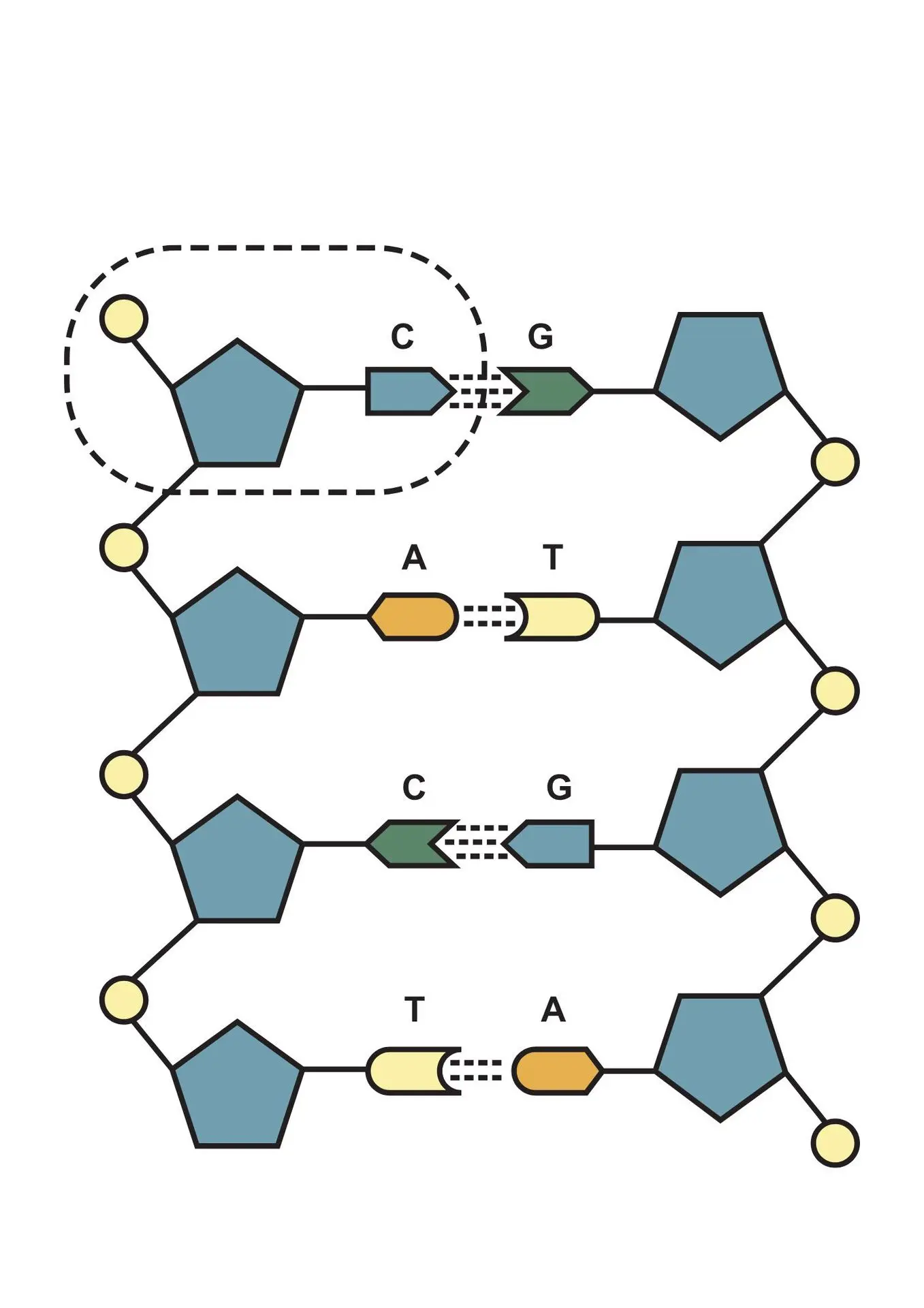
Integrated Platforms for Nucleic Acid Manufacturing and Analysis

Nucleic Acid Manufacturing for Clinical Applications
To support the production of clinical-grade gene therapy vectors, specialized facilities provide end-to-end services for the manufacturing of nucleic acid components such as plasmid DNA, synthetic mRNA, siRNA, and custom oligonucleotides. These components are produced under strict quality frameworks, including GMP-compliant processes when required for clinical applications. Sophisticated purification techniques—such as anion exchange chromatography, endotoxin removal, and sterile filtration—are applied to ensure high purity, integrity, and batch-to-batch reproducibility. This guarantees compatibility with downstream vector development workflows, including AAV, lentiviral, and LNP-based systems.

Advanced Quality Control and Analytical Validation
In parallel, state-of-the-art analytical platforms are employed to verify nucleic acid identity, potency, and safety. Facilities routinely perform critical assessments such as endotoxin quantification, residual host cell DNA/RNA analysis, plasmid topology profiling, and RNA integrity evaluations using validated and regulatory-aligned protocols. These rigorous quality control measures are essential to meet the standards required for investigational new drug (IND) applications and clinical trial use. By integrating manufacturing and analytics under one infrastructure, these platforms streamline the development of next-generation biologics and help ensure safe, effective therapeutic delivery.
Let's get in touch
Connect with your customers to provide better service. You can edit the form fields to get more accurate information.